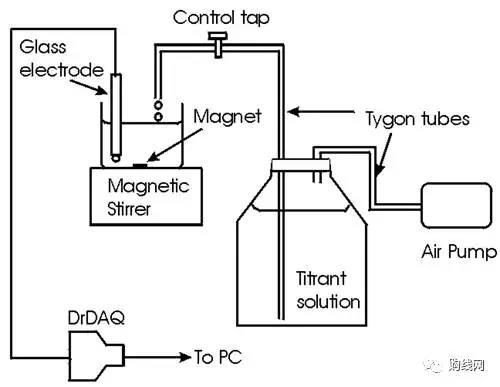
Introduction: The purpose of this experiment is to obtain a titration curve for the acid-base neutralization reaction. It is almost identical to the student's chemistry textbook. The titration curve is a graph showing the change in the pH of the titration solution relative to the volume of the added standard solution (titrant). The acid-base titration curve can be constructed in several ways. One method is to manually record and plot the pH after each manual addition of the aliquot of the titrant solution. Another method is to continuously record and plot the pH automatically during the automatic addition of the titrant. The last method is the operation of expensive automatic titration equipment. The DrDAQ data logger connected to the PC using PicoLog data logging software allows automatic recording and plotting of pH values. Continuous addition of the titrant solution can be achieved by a peristaltic pump or a syringe pump that pumps the solution at a predetermined and fixed rate. A cheaper alternative is to use an air pump (such as used in a home aquarium). The purpose of this experiment is multifaceted: 1. Construct an acid-base titration curve in a very similar manner to that provided by an automated titrator. 2. Learn some of the principles associated with acid-base titration curves by using DrDAQ as a teaching tool. 3. Use the resulting titration curve to determine the concentration of some analytes in common samples, such as acetic acid in vinegar and sodium bicarbonate in baking powder. Equipment required: DrDAQ; pH electrode; one beaker (125 ml); magnetic stirrer - magnetic bar; air pump (JUNACO9903) (can prove the validity of the experiment), higher precision and reliability, preferably peristaltic pump or injection Pump; dropper; 1 liter glass bottle with lid; 0.1 mol/L HCl; 0.1 mol/L NaOH; 0.1 mol/L Na2CO3; acid acid thin; cylinder, 25 ml; 5 ml graduated pipette; 25 ml pipette. Experimental setup: The connection shown in the figure below is shown. The air pump pushes the titrant solution at a fixed and known flow rate: Among them, the volume (V) of the post-added titrant = flow rate (mL / sec) X time (t) (seconds), such that the amount of titrant added becomes a linear function of time, and variables recorded by DrDAQ and PicoLog can be used. The following issues need to be noted: 1. The flow rate remains the same: the speed of the pump; 2. The setting of the control faucet; 3. The height of the tube is higher than the height of the air pump (this is not important for other types of pumps). Ensure that the inlet airflow is in the glass bottle. 4. Do not allow bubbles to enter the titrant solution; 5. Make sure that there is no air leakage around the glass tube in the dropper; 6. It is recommended to use epoxy to seal the pipe on the cap; 7. Use high agitation Rate and place the glass pH electrode as far as possible from the titration drop to minimize the local concentration of the titrant near the glass pH electrode. conduct experiment Note: Remember to wash the reaction beaker and pH electrode with distilled water before each titration. Determination and setting of traffic: 1. Place a 25 ml graduated cylinder at the end of the tube. 2. Turn on the air pump and collect a volume (eg 20 ml) of titrant in the cylinder. The time required to measure (t). 3. Calculate the flow rate (F): F = V (ml) / t (s) A flow rate of about 1-3 ml / min (0.0166 - 0.05 ml / sec) is suitable. Do not change the settings after measuring the flow. Experiment 1: Determination of unknown HCl concentration (normalized HCl) 1. Add an unknown HCl solution (?1 mol/l) to a 1 liter glass vial. 2. Pipette 5 ml of 1.0 mol/L Na2CO3 solution into a 125 ml glass beaker. 3. Add about 50 ml of distilled water. 4. Immerse the glass pH electrode into the solution and turn on the magnetic stirrer. 5. Set PicoLog to monitor the pH of the sample every 2 seconds. At the same time, start recording with DrDAQ and start the flow of the titrant (just turn on the air pump). Note that the initial pH is basic (sodium carbonate is a basic salt). 6. Observe how slowly the pH drops over the entire time interval before the endpoint and how the pH changes abruptly within a very limited time near the endpoint. 7. Observe the advantages of the PicoLog auto-scaling feature in this application. Note that the curves show two pH values ​​at equal time intervals (for equal volume additions). The time (t) required to completely neutralize the sodium carbonate (second endpoint) was measured. The molar concentration (M) of the HCl solution can be calculated by this expression M (HCl) = [(M × V) carbonate × 2] / [(t × F)]. This setup is almost identical to the setting of a commercial automatic titrator, which has an integrated system that provides titrants, monitors pH, plots curves and detects endpoints. Experiment 2: Determination of the concentration of sodium hydroxide solution 1. Transfer 5 ml of unknown sodium hydroxide solution to a 125 ml glass beaker. 2. Add about 50 ml of distilled water. 3. Use the same HCl as the previous part. 4. Repeat the above experimental one related operation. Note: The initial pH is very high (strong base). Only one large pH jump was observed. The time (t) at which the endpoint was found (in this case, the steepest point in the curve corresponding to pH 7). (The PicoLog cursor will help you define the end point.) The molar concentration (M) of the NaOH solution was calculated from the expression of M(NaOH)=[M(HCl)×(t)×F]/V(NaOH). Experiment 3: Determination of sodium bicarbonate content in commercial baking powder 1. Suspend 5 g of baking powder in 100 ml of distilled water. 2. Shake well and transfer the 50 ml aliquot to a 125 ml glass beaker. 3. Perform the above titration using the same HCl solution. Note: The initial pH of the bicarbonate solution is much lower (7.2) than the initial pH of the carbonate solution described in Experiment 1. Calculation of % (w/w) of sodium hydrogencarbonate: sodium hydrogencarbonate % (w/w) = [(M(HCl) × t × F × 84 × 2] × 100/5 Experiment 4: Determination of acetic acid content in vinegar 1. Fill the glass bottle with NaOH solution in Part 3 for use as a titrant. 2. Calibrate the flow rate (F). 3. Transfer 10 ml commercial vinegar to a 125 ml glass beaker. 4. Dilute with about 50 ml of distilled water. Repeat the above experiment-related operation. 5. The initial pH was observed in the acidic region. This is because acetic acid is present in the vinegar. Calculate the concentration of acetic acid: the concentration of acetic acid (w/w) = [(M(NaOH) × F × t × 60.05 × 10] / 1000 Experiment 5: Comparison of titration of acetic acid and HCl with NaOH 1. Pipette 10 ml aliquots of HCl solution in a 125 ml beaker. 2. Dilute with about 50 ml of distilled water. 3. Repeat the relevant operations in Part 2. 4. The initial pH was observed to be in the acidic region and only one pH jump occurred. When using, put the original on the first layer of the copy table, then take the normal A4 printing paper or drawing manuscript on the original, plug in the power supply to light the copy table, press the brightness you want, so it is very clear If you can see the pattern on the original clearly, you can directly copy the pattern according to the pattern. After you draw it, you can have two identical artworks! LED Light Pad,A1 LED Light Pad,Copy Table, Light Transmission Table Guangdong Jishengke Industrial Co.,Ltd , https://www.dgjskpad.com